Development History
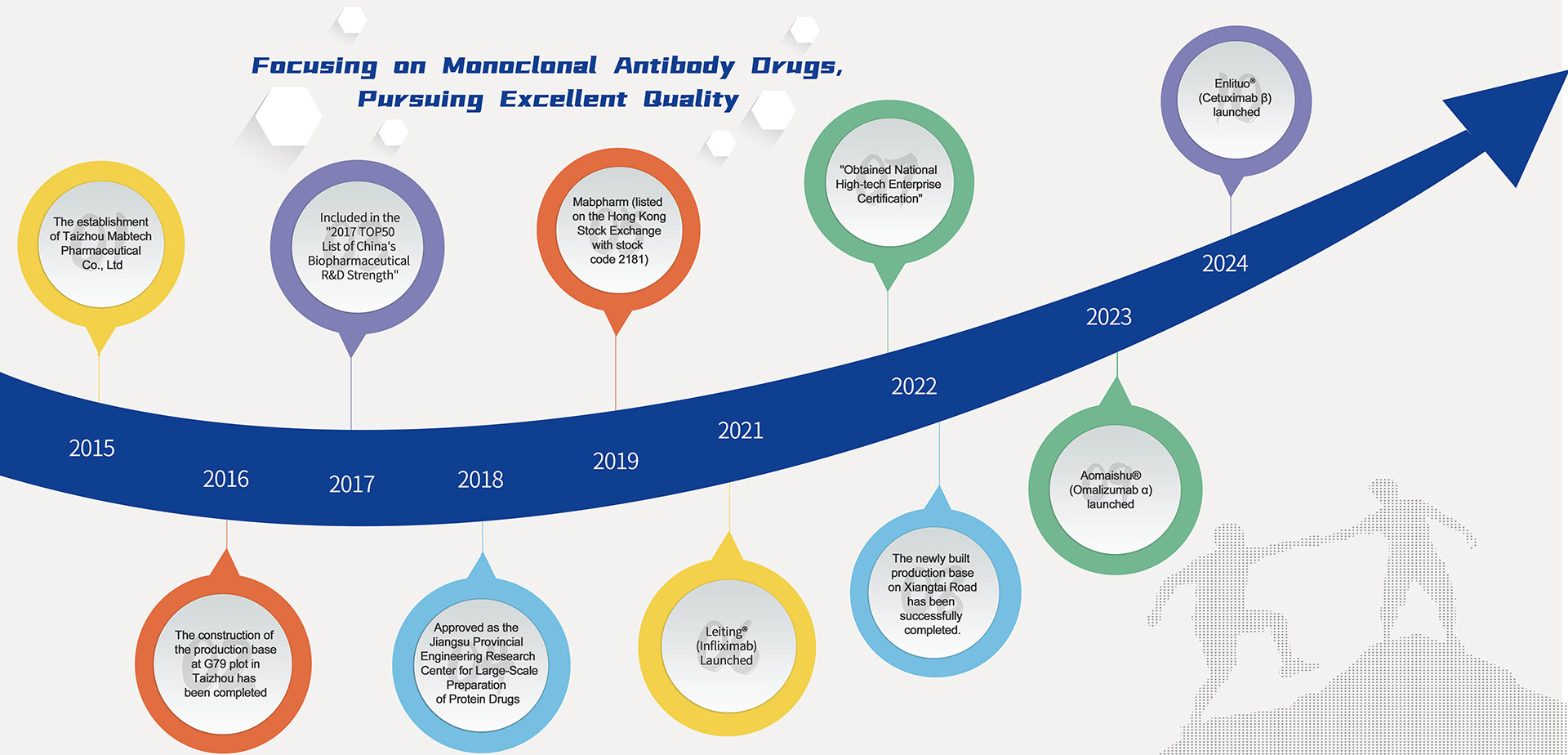
In terms of product R&D, Mabpharm Limited has achieved remarkable results. In July 2021, its biological drug CMAB008 Leiting® (Infliximab for Injection) was successfully approved for marketing, covering multiple indications including but not limited to gastroenterology, rheumatology, and dermatology. Subsequently, the product entered a period of rapid growth in sales and made significant breakthroughs in overseas market expansion. In May 2023, another product CMAB007 Aomaishu® (Omalizumab α for Injection) was also approved for marketing by the National Medical Products Administration, becoming the first domestically produced therapeutic antibody drug for allergic asthma approved in China. In June 2024, the company received more good news as the EGFR antibody drug Enlituo® (Cetuximab β Injection) was approved for marketing,this achievement fills the domestic gap in the field of metastatic colorectal cancer (mCRC) treatment with self-developed EGFR antibody drug products under proprietary intellectual property.
Mabpharm Limited has established a nationwide sales network in China, covering thousands of hospitals at all levels, primary healthcare institutions, and pharmacies across all provinces. In 2024, the company achieved remarkable commercial growth with its revenue increasing by 196.3%. These milestone achievements not only demonstrate Mabpharm Limited's R&D capabilities, but also strengthen the company's reputation in the biopharmaceutical industry, driving its continued growth.
