Overview
Mabpharm Limited focuses on the research, development, and production of new drugs and biosimilars for the treatment of cancer and autoimmune diseases. Through its efficient R&D system and low-cost drug manufacturing capabilities, the company provides high-quality, innovative biopharmaceuticals to benefit a wide range of patients.
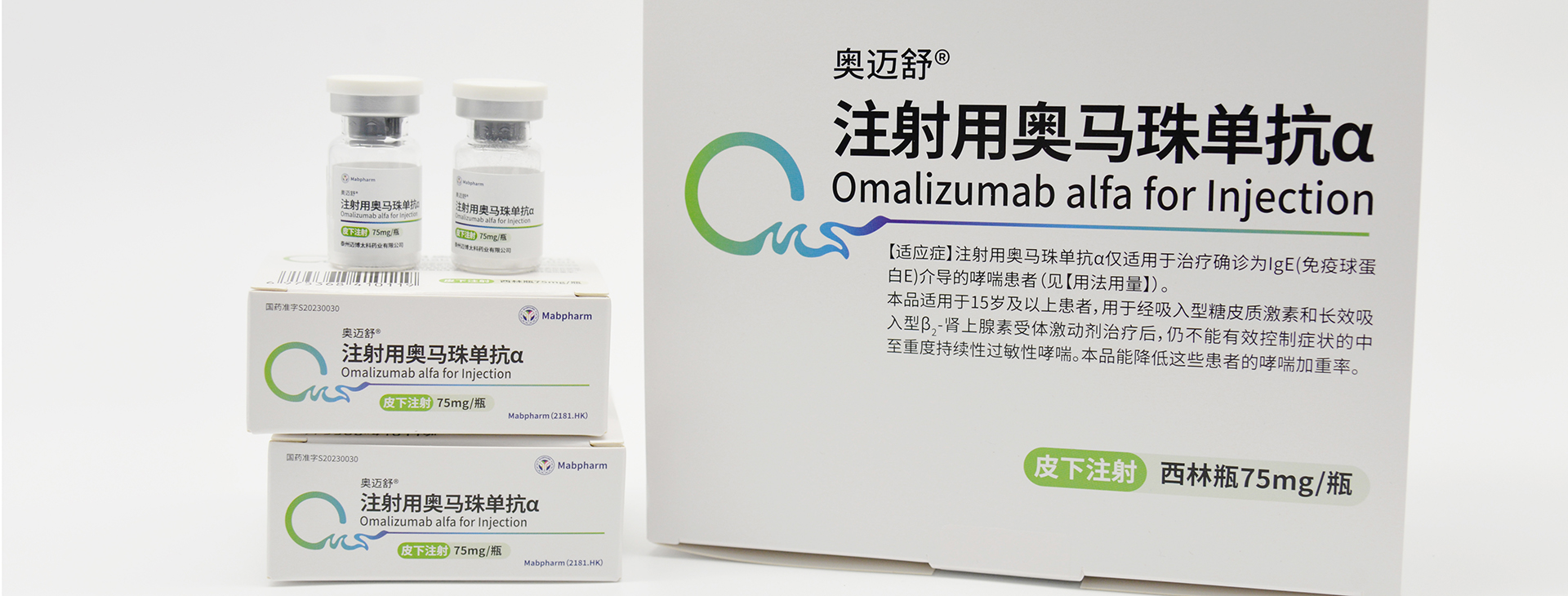
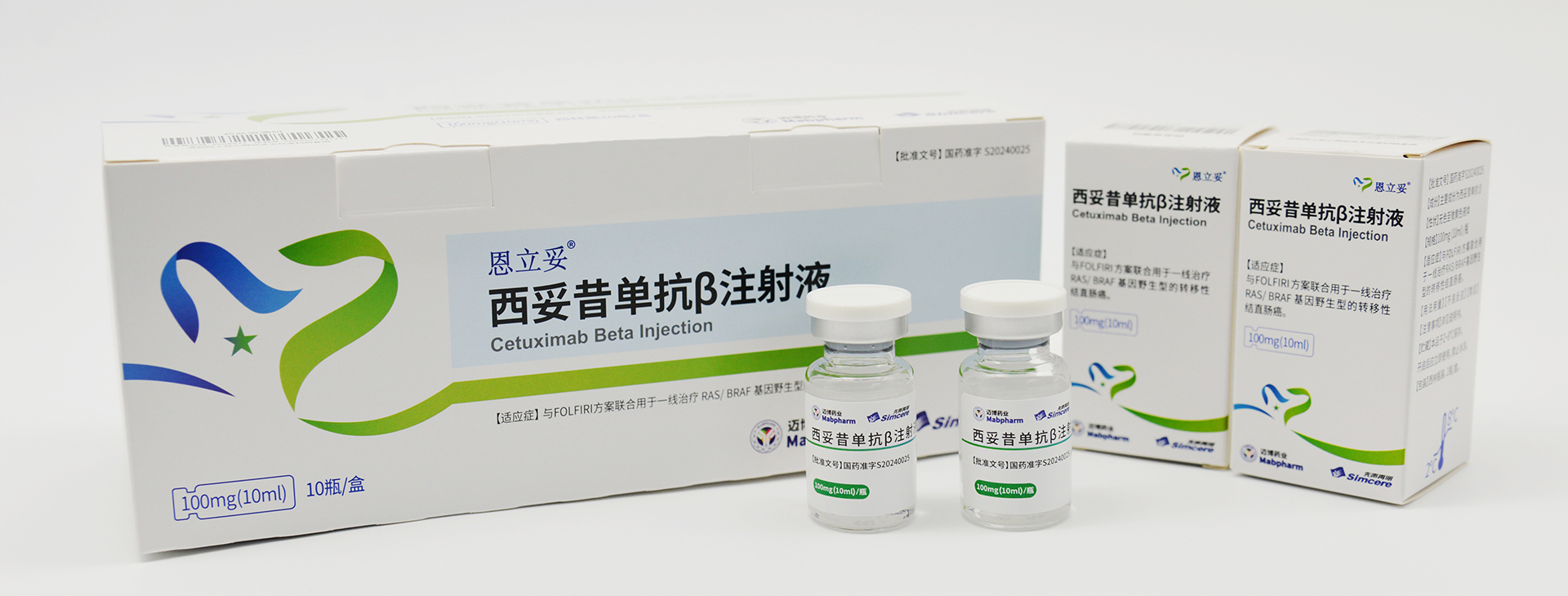
Its product pipeline comprises nine monoclonal antibody drugs and one probody drug, among which CMAB008 Leiting® (Infliximab for Injection), CMAB007 Aomaishu® (Omalizumab α for Injection), and CMAB009 Enlituo® (Cetuximab β Injection) have been approved for marketing. CMAB807/CMAB807X (Denosumab) has completed Phase III clinical trials for osteoporosis and has initiated a multi-indication registration filing pathway according to international regulatory precedents, the candidate drug CMAB015 (Secukinumab) is currently undergoing Phase III clinical studies, and the remaining antibody drugs are in preclinical and early clinical stages of research. Additionally, one monoclonal antibody drug produced under contract has been approved for marketing by the National Medical Products Administration.
 As of December 31, 2024, here is an overview of our drug candidates and their development status:
As of December 31, 2024, here is an overview of our drug candidates and their development status: